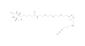product.details.print
Biotin Azide Plus, 5 mg
Molar mass (M) 582,72 g/mol
Storage temp. -20 °C
Transport temp. cooled
WGK 1
Biotin Azide Plus is a biotin probe that enables the possibility for copper-catalyzed Azide-Alkyne Chemistry (CuAAC) to biotin label alkyne or DBCO modified targets. The Azide Plus is a chemical structure that works as a strong copper-chelating system allowing the formation of a strong and active copper complex that makes Biotin Azide Plus the reactant and the catalyst in the CuAAC reaction. Therefore the reaction can be performed under diluted conditions. Once the biotin is attached to a biomolecule fluorescence microscopy detection can be carried out using the well-known signal enhancement strategy of incubation with a fluorescent streptavidin-conjugate.
354,75 €/VE
Excl. btw | 5 mg Per VE
Bestelnr. 259X.1
- Tussentotaal: 0.00
| Bestelnr. | VE | Verp. | Prijs | Hoeveelheid | |
|---|---|---|---|---|---|
| 259X.1 | 5 mg | plastic |
354,75 € |
|
|
| 259X.2 | 10 mg | plastic |
612,75 € |
|
|
|
Op voorraad
Beschikbaar
In bestelling
Niet meer verkrijgbaar
Leveringsdatum onbekend
|
|||||
- Tussentotaal: 0.00
Downloads / MSDS
Algemene informatie
The copper(I)-catalysed azide-alkyne cycloaddition (CuAAC) is the most prominent example of a group of reactions named click reactions. According to Sharpless’ definition, these reactions are characterised by high yields, mild reaction conditions, and by their tolerance of a broad range of functional groups.[1-3] Typically, the reactions require simple or no workup or purification of the product. The most important characteristic of the CuAAC reaction is its unique bioorthogonality as neither azide nor terminal alkyne functional groups are generally present in natural systems.
References:
1 H. C. Kolb, M.G. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. 2001, 40, 2004-2021.
2 C.W. Tornoe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057-3064.
3 V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708-2711; Angew. Chem. Int. Ed. 2002, 41, 2596-2599.
In recent years, click chemistry has become increasingly important and steadily more popular in research and development. The reasons for this are not only the easy handling and the high yields but also the countless application possibilities. Carl ROTH offers you the possibility to purchase the broad portfolio of click reagents, various kits for EdU-based cell proliferation assays and numerous other applications from the well-known brand baseclick. This enables us to offer you a necessary and comprehensive assortment for click chemistry.
Consumer Notice:
▶ aqueous media: copper(II) sulfate 7816, sodium ascorbate 7819, THPTA 7822▶ organic solvents (e.g. DMSO): Copper(I) bromide 7813, TBTA 7814 (copper stabilizing ligand)
Starting from diverse copper catalyst sources, over Cu(I) stabilising ligands to buffer additives and solvents, you will find all necessary reagents in the special ROTI®Click quality to perform your click reactions here.
Analysecertificaten
Type analysis
| Appearance | off-white to slightly orange amorphous solid or oil |
| Purity (HPLC) | ≥95 % |